News Story
NEES project shows hybrid battery/capacitor with off-the-charts cycling capacity
Researchers funded by Nanostructures for Electrical Energy Storage, a DOE Energy Frontier Research Center, have invented a nanowire-based hybrid battery/capacitor that can be recharged hundreds of thousands of times, providing a power source for many applications that would never require replacement. The breakthrough work could lead to commercial batteries with greatly lengthened lifespans for computers, smartphones, cars, and spacecraft.
Scientists have long sought to use nanowires in batteries. While they’re thousands of times thinner than a human hair, they are highly conductive and feature a large surface area for the storage and transfer of electrons. But while they’re high in power, these filaments are extremely fragile and don’t hold up well to repeated discharging and recharging. In a typical lithium ion battery, they expand and grow brittle, which leads to cracking.
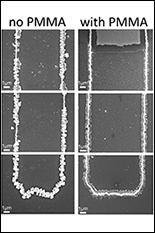
The team, based at the University of California, Irvine, have solved this problem by coating a gold nanowire in a manganese dioxide shell and encasing the assembly in an electrolyte made of a Plexiglas-like gel. The combination is reliable and resistant to failure.
The lead researcher, UCI graduate student Mya Le Thai, charged and discharged the testing electrode up to 200,000 times over three months without detecting any loss of capacity or power, and without fracturing any nanowires. The findings are published today in the American Chemistry Society’s Energy Letters.
Hard work combined with serendipity paid off in this case, said senior author and chair of UCI’s chemistry department Reginald Penner.
"Mya was playing around and she coated this whole thing with a very thin gel layer, and she started to cycle it," said Penner. "She discovered that just by using this gel, the electrode lasted 10 to 20 times as long. She could cycle it 100,000 times without losing any capacity."
He added, "That was crazy, because these things typically die in dramatic fashion after 5,000 or 6,000 or 7,000 cycles at most."
The researchers think the goo plasticizes the metal oxide in the battery and gives it flexibility, preventing cracking.
"The coated electrode holds its shape much better, making it a more reliable option," said Le. "This research proves that a nanowire-based battery electrodes can have a long lifetime, and that in turn, we can make these kinds of batteries a reality."
News Media Contact:
Brian Bell, University of California, Irvine
949-824-8249
bpbell@uci.edu
Published April 20, 2016