News Story
'Fluorinated interphase' bolsters water-based zinc battery chemistry
A research team in the University of Maryland (UMD) Department of Chemical and Biomolecular Engineering (ChBE) has achieved another breakthrough in metallic zinc battery chemistry – after innovating a zinc-air battery cathode reported in Science earlier this year – this time specific to the anode.
The team, led by UMD Professor Chunsheng Wang, created a fluorinated interphase, which enables reversible water-based zinc battery chemistries. Longsheng Cao (ChBE Post-doc), Dan Li (ChBE Post-doc) and Travis Pollard (U.S. Army Research Lab) served as first authors on the study, published in Nature Nanotechnology on May 10.
"Metallic zinc is a supreme anode because it boasts high capacity, low redox potential, high abundance and low toxicity," said Cao. "It's also incredibly safe, but suffers from severe irreversibility -- for example, we often see dendrite growth and low coulombic efficiency in aqueous electrolytes, which makes the formation of a solid-electrolyte interphase [SEI] impossible."
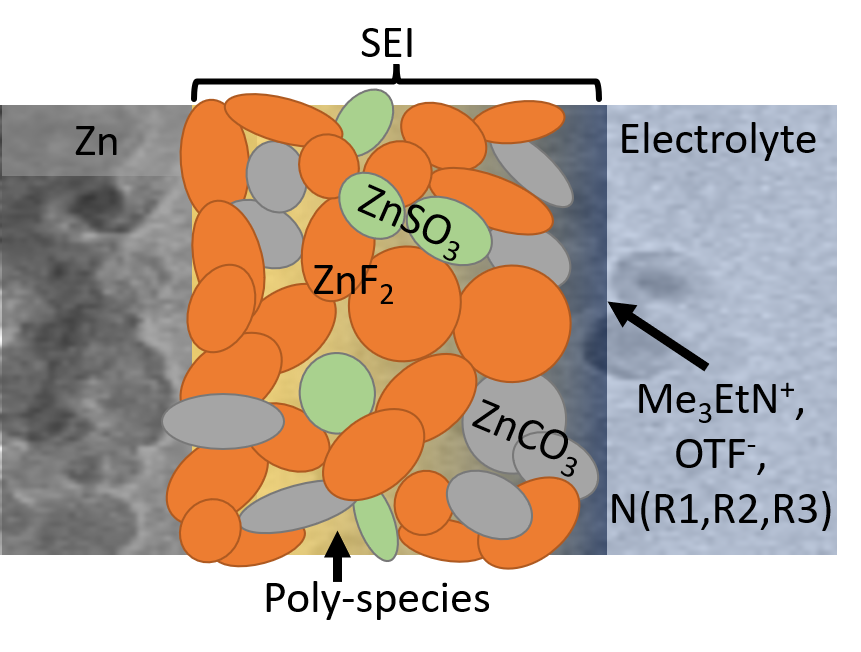
To that end, the Wang group created a diluted and acidic water electrolyte, with an alkylammonium salt additive, which gave way to the formation of a robust and waterproof SEI. This chemistry offers dendrite-free zinc plating and stripping at nearly 100% coulombic efficiency.
The SEI, mainly composed of hydrophobic inorganic fluoride, allows zinc ions to move back and forth, blocks water penetration, and prevents electron transfer. Such chemistry prohibits electrolyte or zinc anode consumption, which enables long-term use of aqueous zinc batteries.
This study continues research progress in the zinc battery including air cathode, electrolytes, organic electrolyte coating, SEI design, and MnO2 cathodes.
For additional information:
Cao, L., Li, D., Pollard, T., Deng, T., Wang, C. et al. (10 May 2021). Fluorinated interphase enables reversible aqueous zinc battery chemistries, Nature Nanotechnology. DOI: 10.1038/s41565-021-00905-4.
Published May 10, 2021