News Story
High Temperature Thermal Shocks Increase Stability of Single Atom Catalysts
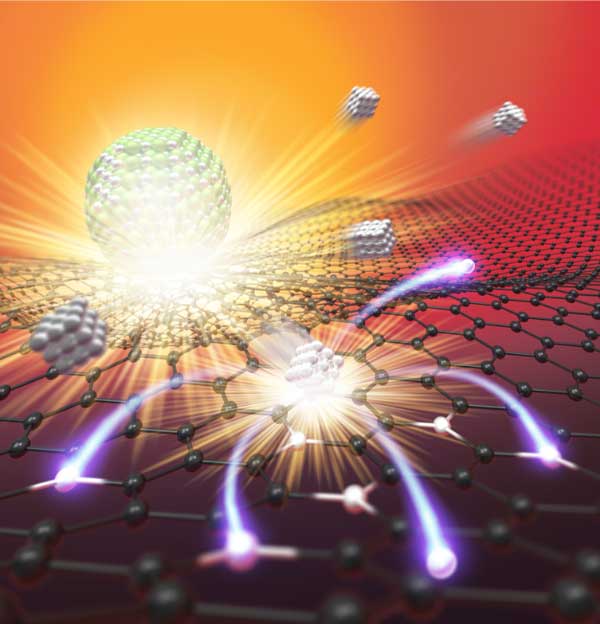
Image created by Jiaqi Dai for UMD.
Catalysts are essentially boosters that increase the rate of a chemical reaction, and are widely used in the fields of petroleum refining, coal and natural gas conversion, and ammonia production, to name a few. Catalysts also drive emerging battery and fuel cell technology, which is usually (thermally or electrically) energy-intensive, thus requiring catalysis to reduce the reaction temperature, pressure, or electrochemical over-potentials.
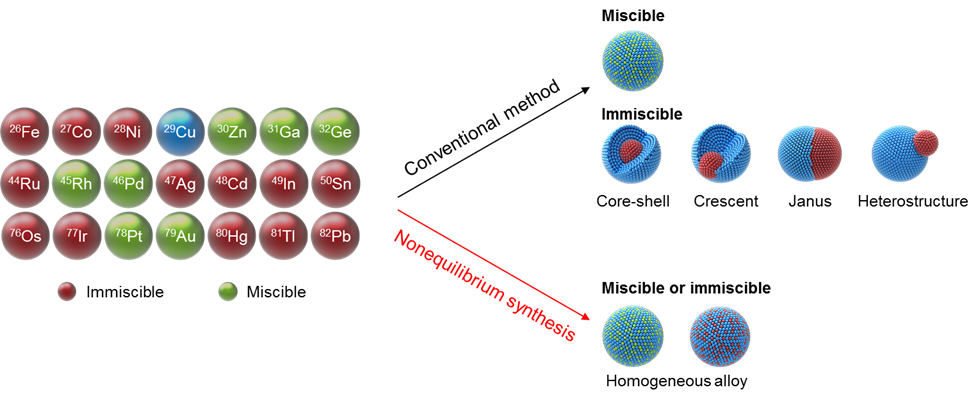
Single atom catalysts maximize the metal utilization efficiency of each atom and provide superior performance, representing the frontier of catalysis. Single atoms, however, are typically unstable when synthesized at low temperature (e.g., less than 1000K), and tend to re-aggregate into nanoparticles as a means of minimizing surface energy. To that end, a research team in the University of Maryland (UMD) Department of Materials Science and Engineering (MSE) developed a high temperature shockwave catalysis method - reaching up to 3000K, which is half the temperature of the sun - intended to "anchor" single atoms onto the substrate, offering superior thermal stability.
The research team led by MSE Professor Liangbing Hu, published their study in Nature Nanotechnology on August 12. Yonggang Yao, MSE Ph.D. Student and member of Dr. Hu's research team, served as the lead author on the paper.
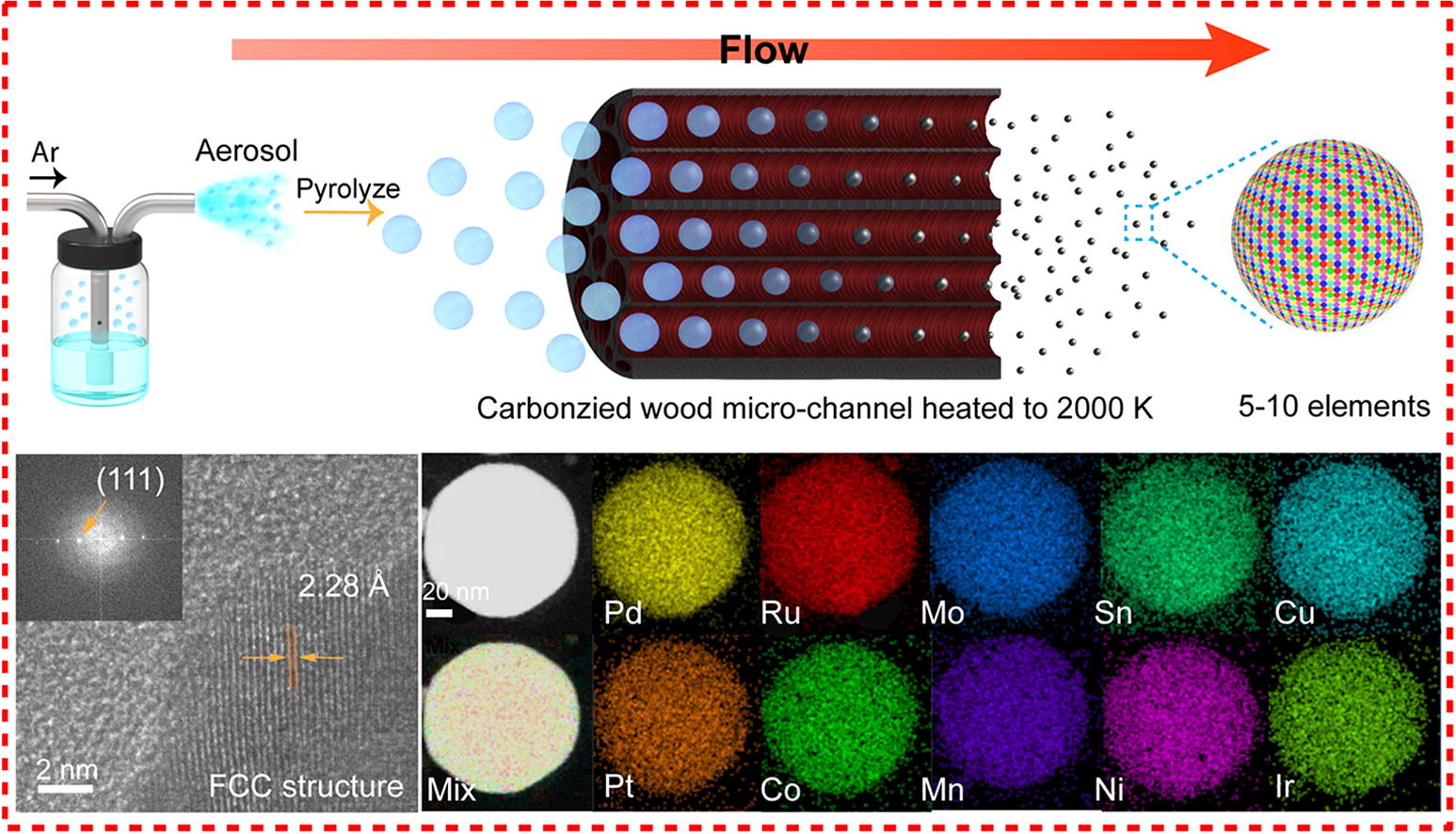
"Our method is achieved using periodic on-off heating featuring a short on-state (~1500K for 55 ms) and a 10-times longer off-state (room temperature)," said Yao. "The high temperature provides activation energy for atom dispersion by forming strong metal-defect bonds, while the off-state critically ensures the overall stability."
Joule heating was utilized to hit the high temperature marks, and the team confirmed synthesis using in situ scanning transmission electron microscopy (STEM). This technique can be used in catalytic reactions such as methane conversion, which converts natural gas into useful chemicals such as ethylene, ethane and benzene.
“The reported shockwave method is facile, ultrafast and universal, which opens a general route for single atom manufacturing that is conventionally challenging," said Hu.
This study was a multi-institutional collaboration including the Reza Shahbazian-Yassar group at the University of Illinois, Chicago; Chao Wang's group at John Hopkin's University; Teng Li's group in the UMD Department of Mechanical Engineering; the Tianpin Wu and Jun Lu group at Argonne National Laboratory; Chongmin Wang's group at Pacific Northwest National Laboratory, and Michael Zachariah's group at UMD.
For additional information:
Nature Nanotechnology, 2019, DOI: 10.1038/s41565-019-0518-7
Published August 12, 2019