Press Release
Sulfur Provides Promising 'Next-Gen' Battery Alternative
Multi-institutional research team led by Chunsheng Wang published in PNAS.
FOR IMMEDIATE RELEASE June 15, 2020
CONTACT:
Katie Holland
301 405 0379
khollan3@umd.edu
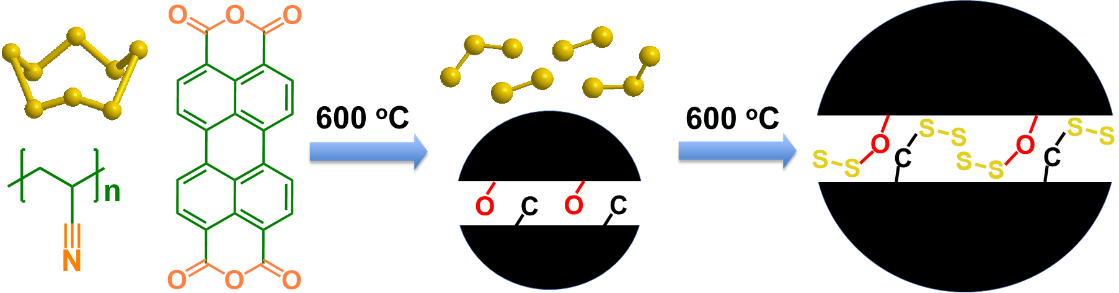
Image: A schematic illustration of the formation of chemical bonding stabilized carbon-small sulfur composite (provided by C. Luo).
With the increasing demand for affordable and sustainable energy, the ongoing development of batteries with a high energy density is vital. Lithium-sulfur batteries have attracted the attention of academic researchers and industry professionals alike due to their high energy density, low cost, abundance, nontoxicity and sustainability. However, Li-sulfur batteries tend to have poor cycle life and low energy density due to the low conductivity of sulfur and dissolution of lithium polysulfide intermediates in the electrolytes, which are generated when pure sulfur reacts with Li-ions and electrons.
To circumvent these challenges, a multi-institutional research team led by Chunsheng Wang at the University of Maryland has developed a new chemistry for a sulfur cathode, which offers increased stability and higher energy of Li-sulfur batteries. Chao Luo - an assistant professor of chemistry and biochemistry at George Mason University - served as first author on the study, published in the Proceedings of the National Academy of Sciences (PNAS) on June 15.
Numerous conductive materials such as graphene, carbon nanotube, porous carbon and expanded graphite were used to prevent the dissolution of polysulfides and increase the electrical conductivity of sulfur cathodes - the challenge here is encapsulating the nano-scale sulfur in a conductive carbon matrix with a high sulfur content to avoid the formation of polysulfides.
"We used the chemical bonding between sulfur and oxygen/carbon to stabilize the sulfur," Luo said. "This included a high temperature treatment to vaporize the 'pristine' sulfur and carbonize the oxygen-rich organic compound in a vacuum glass tube to form a dense oxygen-stabilized sulfur/carbon composite with a high sulfur content."
In addition, scanning electron microscope (SEM) and transmission electron microscopy (TEM) instruments, X-ray photoelectron spectroscopy (XPS) and pair distribution function (PDF) were used to illustrate the reaction mechanism of the electrodes.
"In the dense S/C composite materials, the stabilized sulfur is uniformly distributed in carbon at the molecular level with a 60% sulfur content," Wang said. "The formation of solid electrolyte interphase during the activation cycles completely seal the sulfur in a carbon matrix, offering superior electrochemical performance under lean electrolyte conditions."
Li-sulfur batteries have applications in household and handheld electronics, electric vehicles, large scale energy storage devices and beyond.
For additional information:
Luo, C. et al. (15 June 2020). A chemically stabilized sulfur cathode for lean electrolyte lithium sulfur batteries. PNAS. DOI: 10.1073/pnas.2006301117
About the A. James Clark School of Engineering
The University of Maryland’s A. James Clark School of Engineering is a premier program, ranked among the top 20 in the world. Located just a few miles from Washington, D.C., the Clark School is at the center of a constellation of high-tech companies and federal laboratories, offering students and faculty access to unique professional opportunities.
Our broad spectrum of academic programs, including the world’s only accredited undergraduate fire protection engineering program, is complemented by a vibrant entrepreneurial ecosystem, early hands-on educational experiences, and participation in national and international competitions.
The Clark School is leading research advancements in aerospace, bioengineering, robotics, nanotechnology, disaster resilience, energy and sustainability, and cybersecurity. From the universal product code to satellite radio, SMS text messaging to the implantable insulin pump, our students, faculty, and alumni are engineering life-changing innovations for millions. Learn more at www.eng.umd.edu.